chimeraviz is an R package that automates the creation of chimeric RNA visualizations.
If you wish to cite chimeraviz, please cite this application note.
Previous citations:
- Alternative PDGFD rearrangements in dermatofibrosarcomas protuberans without PDGFB fusions
- Transcriptional-Readthrough RNAs Reflect the Phenomenon of “A Gene Contains Gene(s)” or “Gene(s) within a Gene” in the Human Genome, and Thus Are Not Chimeric RNAs
- FuSpot: a web-based tool for visual evaluation of fusion candidates
- Clinker: visualizing fusion genes detected in RNA-seq data
- Integrative analysis of DNA and RNA sequencing data from cancer
- Clinicopathologic Features of CIC-NUTM1 Sarcomas, a New Molecular Variant of the Family of CIC-Fused Sarcomas
- Clinicopathologic and Molecular Features of a Series of 41 Biphenotypic Sinonasal Sarcomas Expanding Their Molecular Spectrum
- Genomic Profiling Of Primary Histiocytic Sarcoma Reveals Two Molecular Subgroups
- Using R and Bioconductor in Clinical Genomics and Transcriptomics
- annoFuse: an R Package to annotate and prioritize putative oncogenic RNA fusions
- CRTC1-TRIM11 fusion defined melanocytic tumors: A series of four cases
- Detailed Molecular and Immune Marker Profiling of Archival Prostate Cancer Samples Reveals an Inverse Association between TMPRSS2:ERG Fusion Status and Immune Cell Infiltration
- FGviewer: an online visualization tool for functional features of human fusion genes
- Molecular characterization of organoids derived from pancreatic intraductal papillary mucinous neoplasms
- RNA-sequencing of IDH-wild-type glioblastoma with chromothripsis identifies novel gene fusions with potential oncogenic properties
- Immuno-genomic classification of colorectal cancer organoids reveals cancer cells with intrinsic immunogenic properties associated with patient survival
- Fusion Genes in Prostate Cancer: A Comparison in Men of African and European Descent
- Integrative multi-omics analysis reveals molecular subtypes and tumor evolution of synovial sarcoma
- MALAT1 Fusions and Basal Cells Contribute to Primary Resistance against Androgen Receptor Inhibition in TRAMP Mice
- Characterization of a castrate-resistant prostate cancer xenograft derived from a patient of West African ancestry
- Novel transcription-induced fusion RNAs in prostate cancer
- An organoid biobank for childhood kidney cancers that captures disease and tissue heterogeneity
- Analyses transcriptomiques et fonctionnelles des anomalies moléculaires récurrentes des sarcomes du stroma endométrial
- Genomic landscape of uveal melanoma
- Targeted in silico characterization of fusion transcripts in tumor and normal tissues via FusionInspector
- Detección de fusiones génicas en datos de secuenciación de RNA en muestras de pacientes con melanoma lentiginoso acral del INCan-México
- Comparative transcriptome characterization of esophageal squamous cell carcinoma and adenocarcinoma
The following is an excerpt of the package vignette.
chimeraviz allows you to import data from these different fusion-finders:
- deFuse
- EricScript
- InFusion
- JAFFA
- FusionCatcher
- FusionMap
- PRADA
- SOAPFuse
- STAR-FUSION
- ChimPipe
- SQUID
- Aeron
Getting started is easy:
# Load chimeraviz
library(chimeraviz)
# Get reference to results file from deFuse
defuse833ke <- system.file(
"extdata",
"defuse_833ke_results.filtered.tsv",
package="chimeraviz")
# Load the results file into a list of fusion objects
fusions <- import_defuse(defuse833ke, "hg19")
Import functions for the other supported fusion-finders are similarly named (for example import_eriscript or import_infusion).
A list of Fusion objects, objects that represent each fusion transcript, is now available in the list fusions.
length(fusions)
## [1] 17
As you can see, this list has 17 fusion objects. It is straightforward to find a specific fusion event and print information about it, or about each of the partner genes.
# Find a specific fusion event
fusion <- get_fusion_by_id(fusions, 5267)
# Show information about this fusion event
fusion
## [1] "Fusion object"
## [1] "id: 5267"
## [1] "Fusion tool: defuse"
## [1] "Genome version: hg19"
## [1] "Gene names: RCC1-HENMT1"
## [1] "Chromosomes: chr1-chr1"
## [1] "Strands: +,-"
## [1] "In-frame?: FALSE"
# Show information about the upstream fusion partner
upstream_partner_gene(fusion)
## [1] "PartnerGene object"
## [1] "Name: RCC1"
## [1] "ensemblId: ENSG00000180198"
## [1] "Chromosome: chr1"
## [1] "Strand: +"
## [1] "Breakpoint: 28834672"
# Show information about the downstream fusion partner
downstream_partner_gene(fusion)
## [1] "PartnerGene object"
## [1] "Name: HENMT1"
## [1] "ensemblId: ENSG00000162639"
## [1] "Chromosome: chr1"
## [1] "Strand: -"
## [1] "Breakpoint: 109202584"
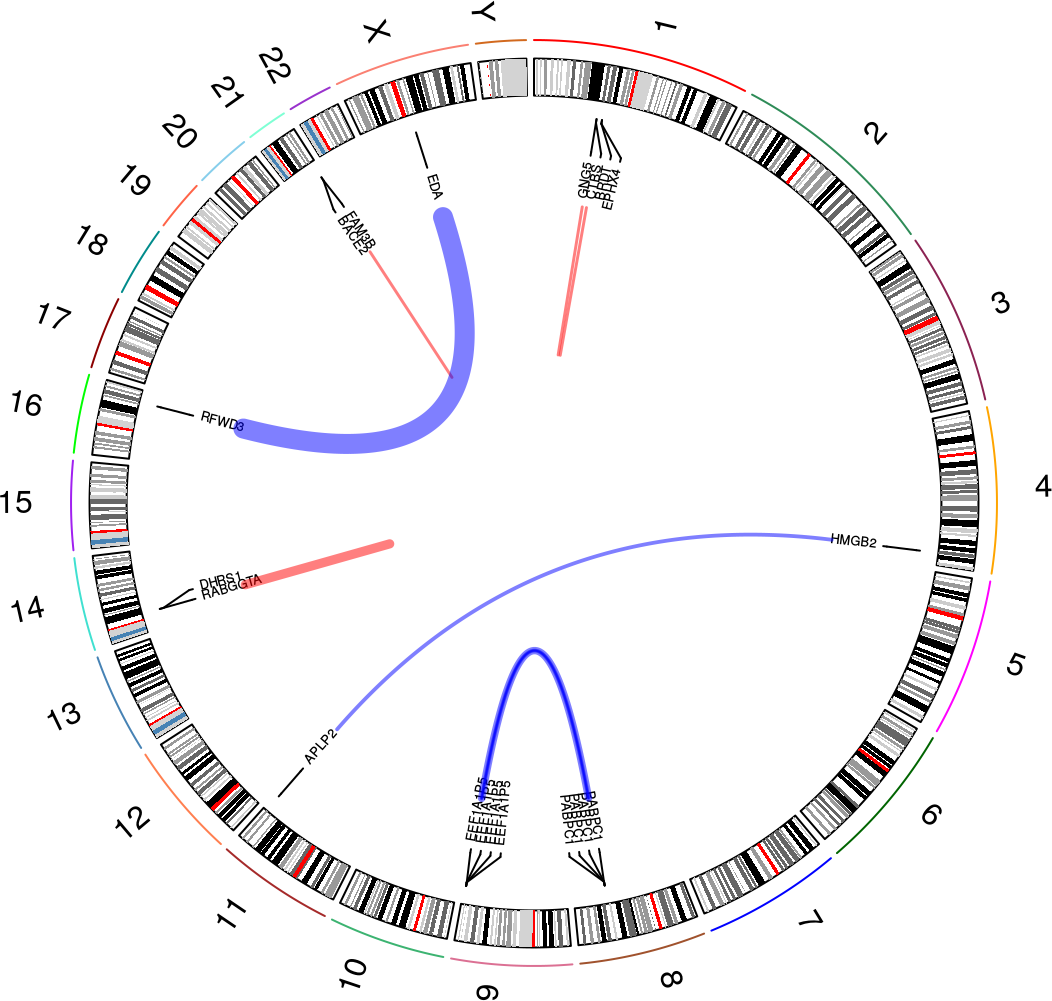
The overview plot is a nice way to get an overview over the nominated fusions in a sample. It will produce a circular plot like this one:
In this plot, you can see the following:
- All chromosomes with cytoband information
- Fusion events as links between locations in chromosomes (with gene names, if there is enough space in the plot)
- Red links indicate intrachromosomal fusions, while blue links indicate interchromosomal fusions
- The widths of each link varies according to how many reads support the fusion event. See the
plot_circle()documentation for more detail on how this is computed
See how to build this plot in the package vignette: Building the overview plot.
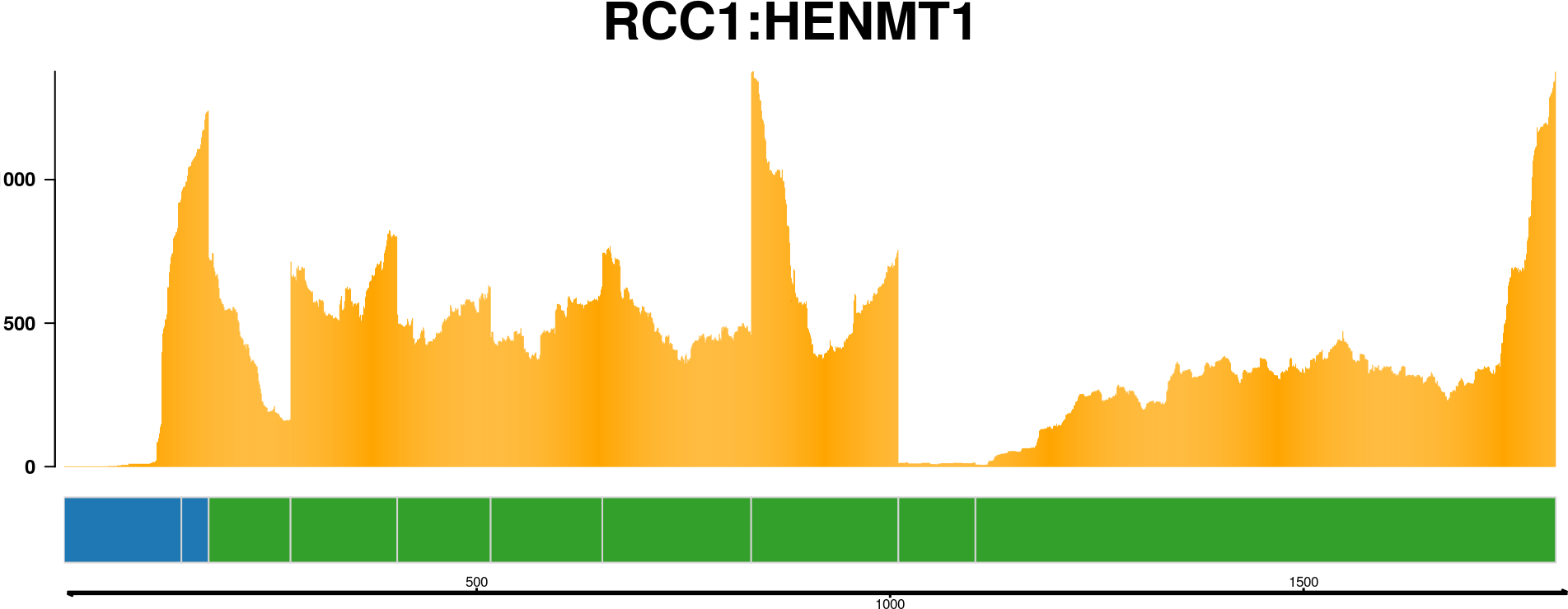
The fusion reads plot is a way to visualize the reads supporting a fusion event mapped to the putative fusion sequence. Many fusion finders report a putative fusion sequence, and by mapping reads to this sequence visualize how well the fusion event is supported. The function plot_fusion_reads() will, given enough data, produce a plot like this one:
As seen in the plot (you might want to open the image in a new tab and zoom in), this fusion event is supported by 6 paired end reads.
See how to build this plot in the package vignette: Building the fusion reads plot.
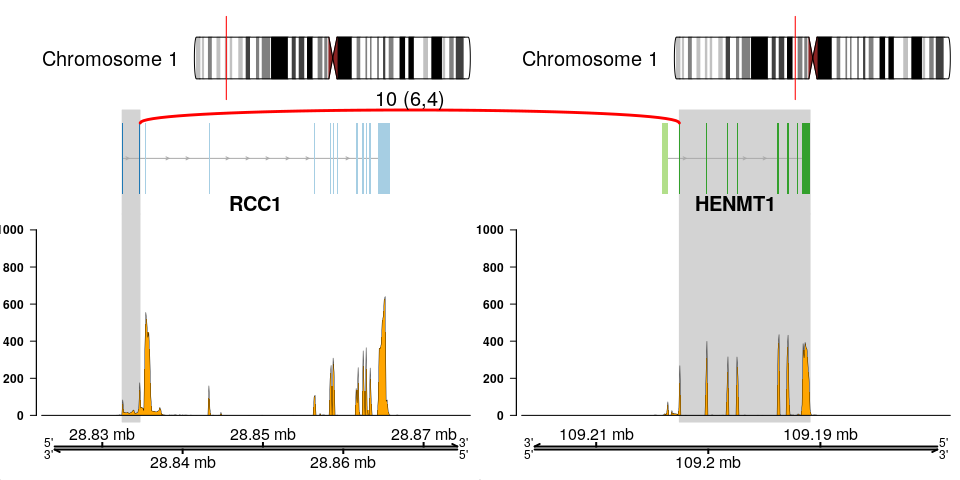
The fusion plot is the main product of chimeraviz, created with the plot_fusion function. It will create a plot like this one:
Or, alternatively:
This plot holds a lot of information. You can see:
- Chromosome ideograms with a red line indicating the location of the two partner genes
- Transcript information showing exons in each partner gene
- The breakpoint between the partner genes, indicated with a red link
- Above the red link you can see the number of sequencing reads that support the fusion event
- Coverage information indicating the RNA expression level
- Genome coordinates (mega basepairs from the p-telomere)
The fusion you can see above is the RCC1-HENMT1 fusion described by Andreas M. Hoff et al. in the paper Identification of Novel Fusion Genes in Testicular Germ Cell Tumors (Cancer Research, 2016).
Note that the plot reverses genes as necessary, so that the fused genes are plotted in the "correct" (5'-to-3') relative orientation.
See how to build this plot in the package vignette: Building the fusion plot.
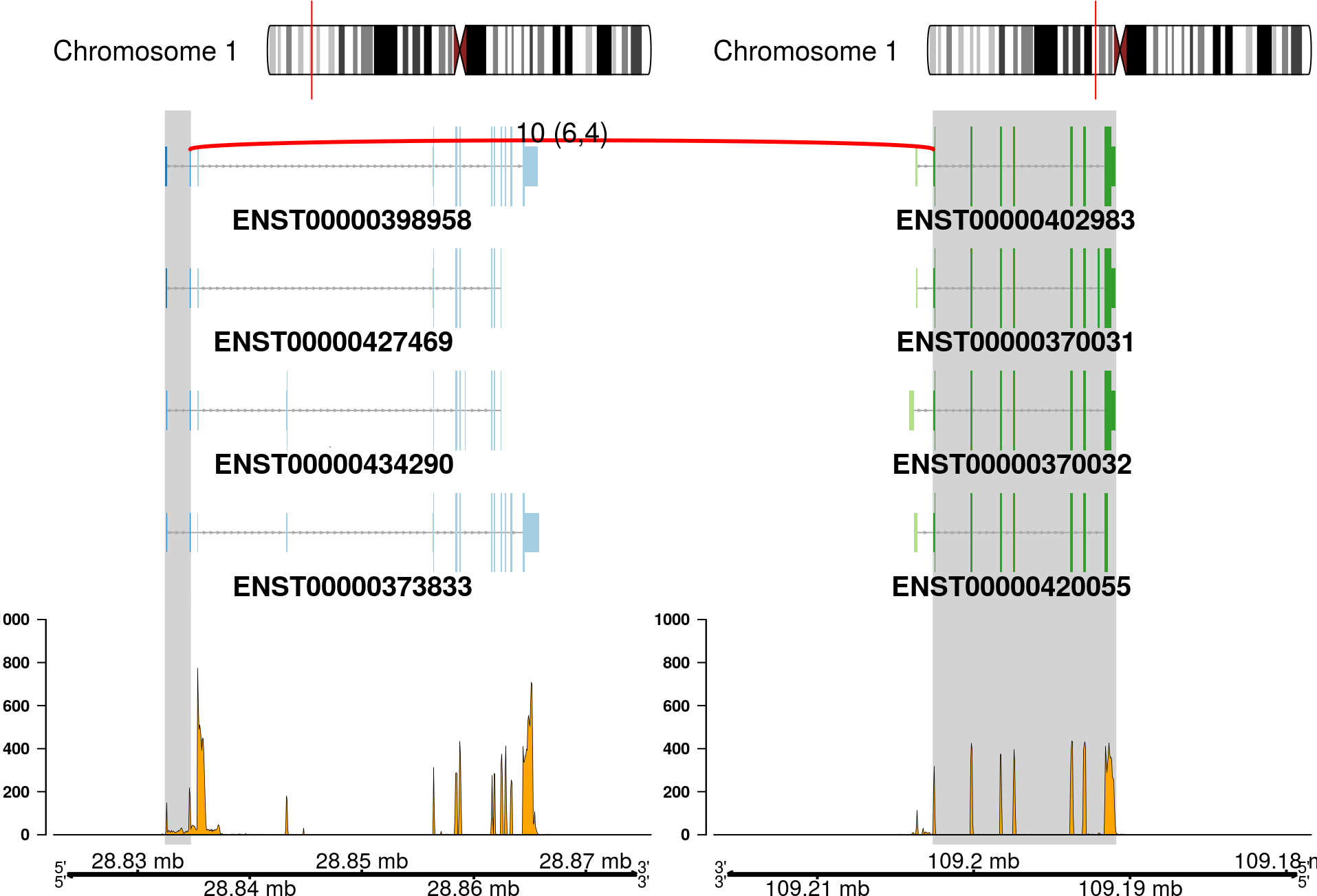
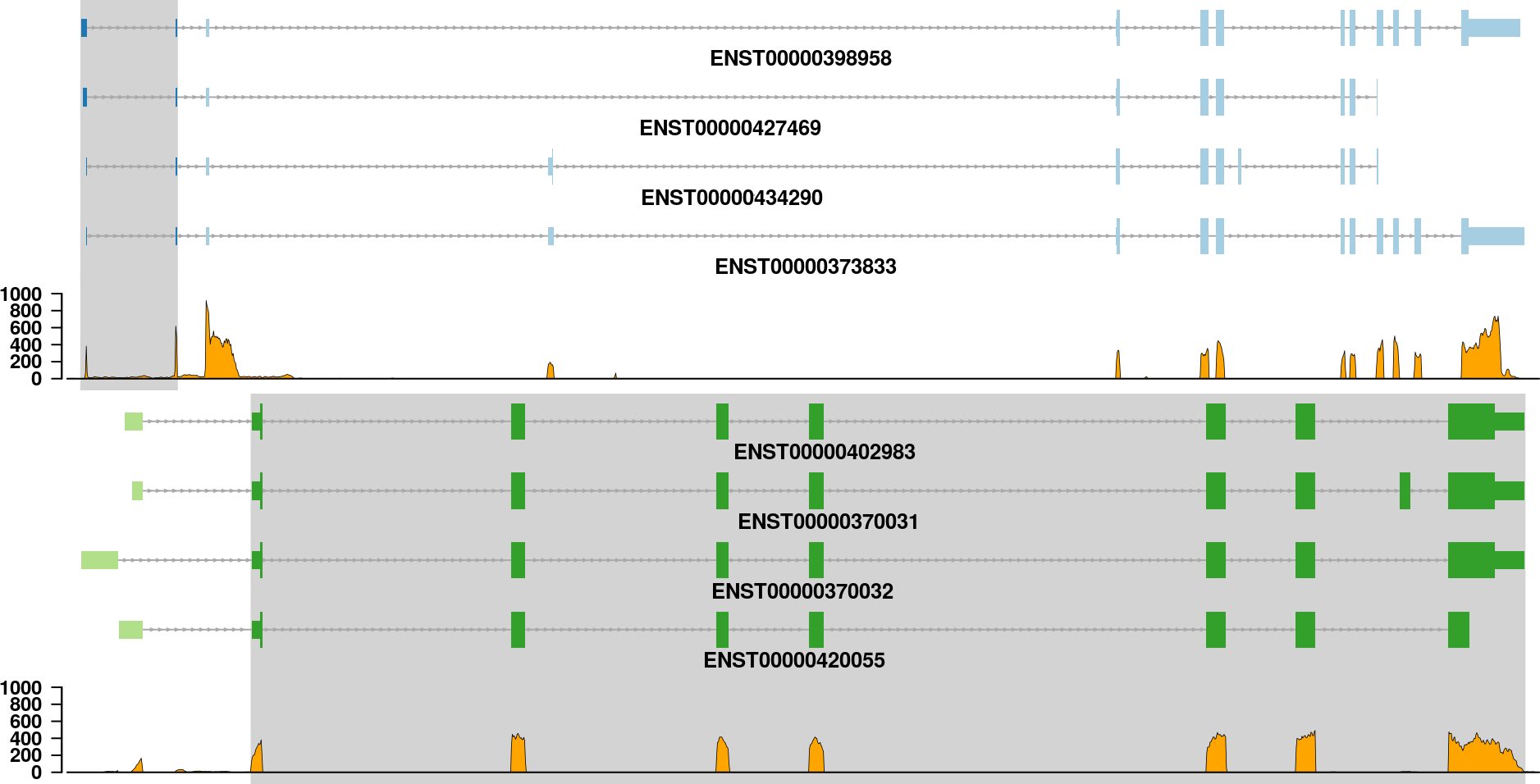
If you are only interested in the transcripts of each partner gene in a fusion event, then it is not necessary to show all the information that is in the fusion plot. The transcripts plot is designed to give a simple view on the transcripts that might be included in the fusion transcript:
See how to build this plot in the package vignette: Building the fusion transcripts plot.
The fusion transcript plot shows the reduced version of all exons that could be part of a fusion transcript. This is a way to view all the possible parts of a fusion transcript merged into one.
See how to build this plot in the package vignette: Building the fusion transcript plot.
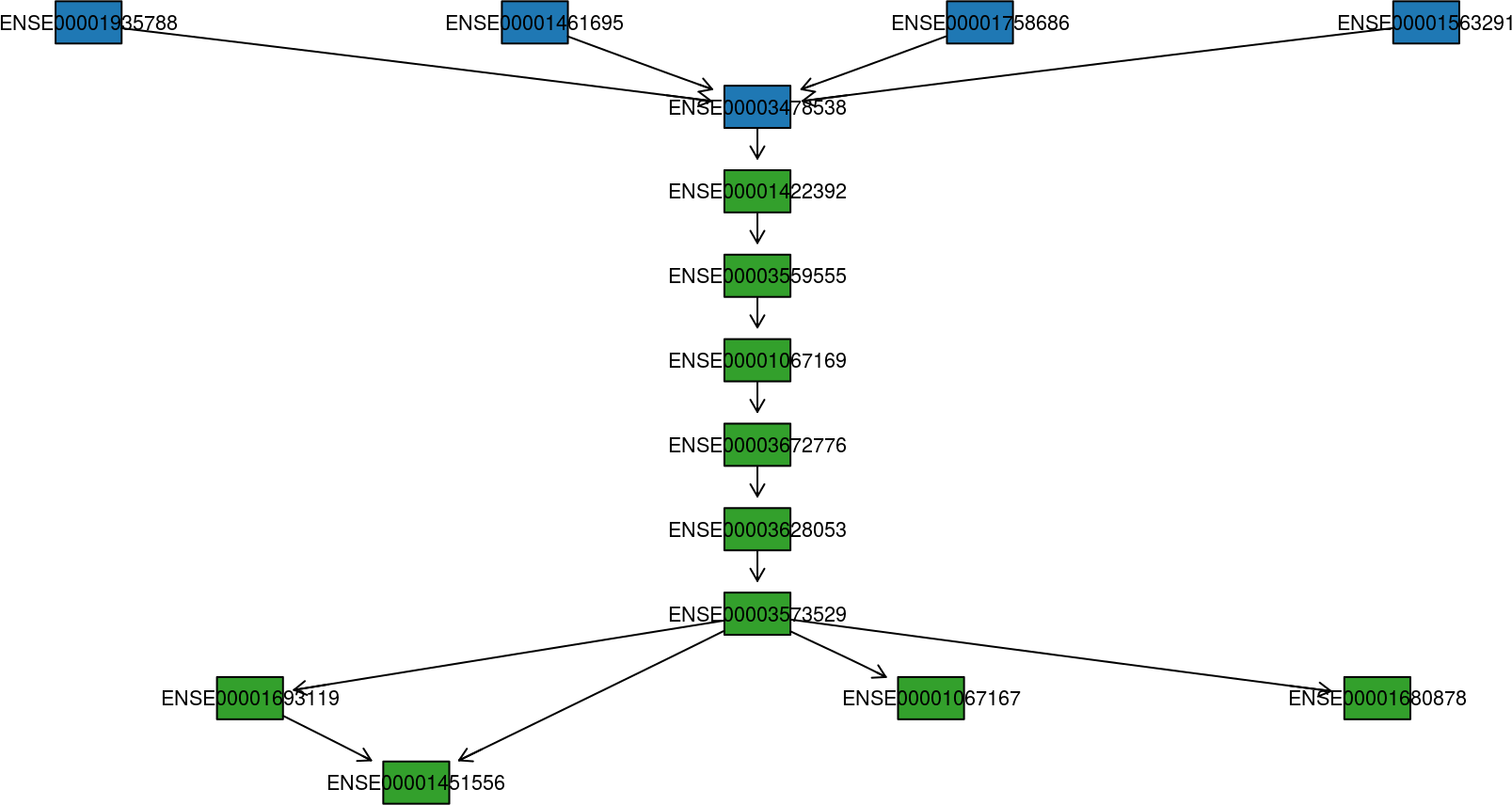
All that is known about a fusion event is that a fusion-finder has scored a possible link between two genes. If there are four transcript variants of the upstream gene partner and four transcript variants of the downstream gene partner, then there are in total sixteen different splice variants of the finally processed fusion transcript. And that is only if we count the known, annotated variants of each gene. How can we make sense of all this? Plotting the transcript together as in previous plots helps, but there is a better way to visualize the putative fusion transcript: As a graph. By representing transcripts as a graph, with exons as nodes and splice junctions as edges, it is much easier to get a view on the putative fusion transcript.
See how to build this plot in the package vignette: Building the fusion transcript plot.
The fusion transcript plot with protein domain annotations shows a specific fusion transcript along with protein domain annotation data. If a bamfile is specified, the fusion transcript will be plotted with coverage information as well.
See how to build this plot in the package vignette: Building the fusion transcript plot with protein domain annotations.
chimeraviz is a Bioconductor package, and is most easily installed via Bioconductor. Please follow the installation instructions here.
Since BioConductor packages are also available as BioConda packages, chimeraviz is also available there: https://anaconda.org/bioconda/bioconductor-chimeraviz.
NOTE: You will likely not get the latest version of chimeraviz if you use the BioConda package. For the latest and greatest, please install from BioConductor.
BioConda packages are also available as BioContainers, so chimeraviz is available as a Docker container. See https://quay.io/repository/biocontainers/bioconductor-chimeraviz?tab=tags.
NOTE: You will likely not get the latest version of chimeraviz if you use the BioContainers Docker image. For the latest and greatest, please install from BioConductor.
If you want to build the package yourself you can follow these instructions:
- Install the dependencies listed under
Imports,Depends, andSuggestsin the package DESCRIPTION file. - Run this command to install the package:
devtools::install_github(
"stianlagstad/chimeraviz",
build_vignettes = TRUE)
Please create an issue on Github if you have any problems at all.
The easiest way to get started working on chimeraviz is by using Docker. See the README.md file in the docker/ folder in this repository for more information.
You can also work on chimeraviz within a Conda environment. Here are the steps to follow:
- Install
conda: Following instructions here. - Run the command
conda env create -n chimeraviz_release -f environment.ymlfrom the root of this repository. - Run the command
conda activate chimeraviz_releaseto activate the conda environment.
Tests are written with testthat and are located in tests/testthat. They can be run with devtools::test() if you have cloned this repository, i.e. not installed the package with devtools::install_github() but have used git clone [email protected]:stianlagstad/chimeraviz.git chimeraviz.
This package was developed by Stian Lågstad for his master thesis: Visualizing chimeric RNA. The work was supervised by Rolf Skotheim and Ole Christian Lingjærde.
The thesis is available here. The application note in Oxford Bioinformatics is available here.